Bone development and disease
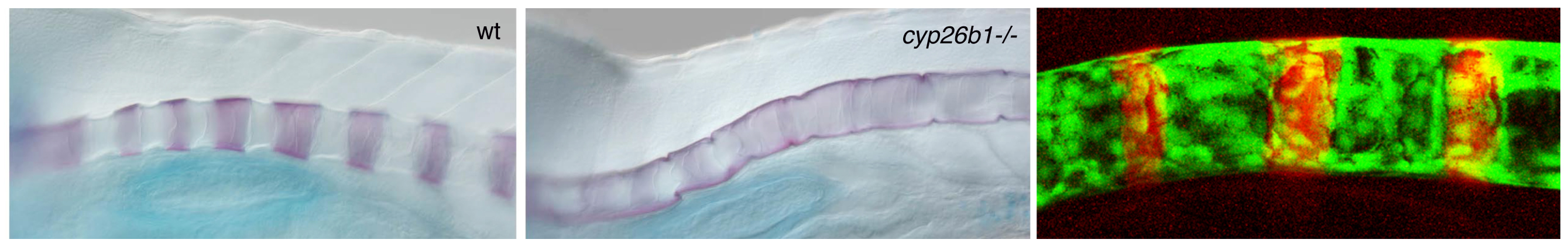
BMPs were initially discovered based on their effect to induce ectopic ossification when applied to skeletal muscle. In zebrafish larvae, transgenic overexpression of BMPs causes enhanced maturation of bone-forming osteoblasts, hyperossification and fusions of skeletal elements, including fusions of the vertebra precursors (centra) of the developing spine. Very similar defects occur in zebrafish mutants in the retinoic acid (RA)-metabolizing enzyme Cyp26b1 (Laue et al., Development 2008).
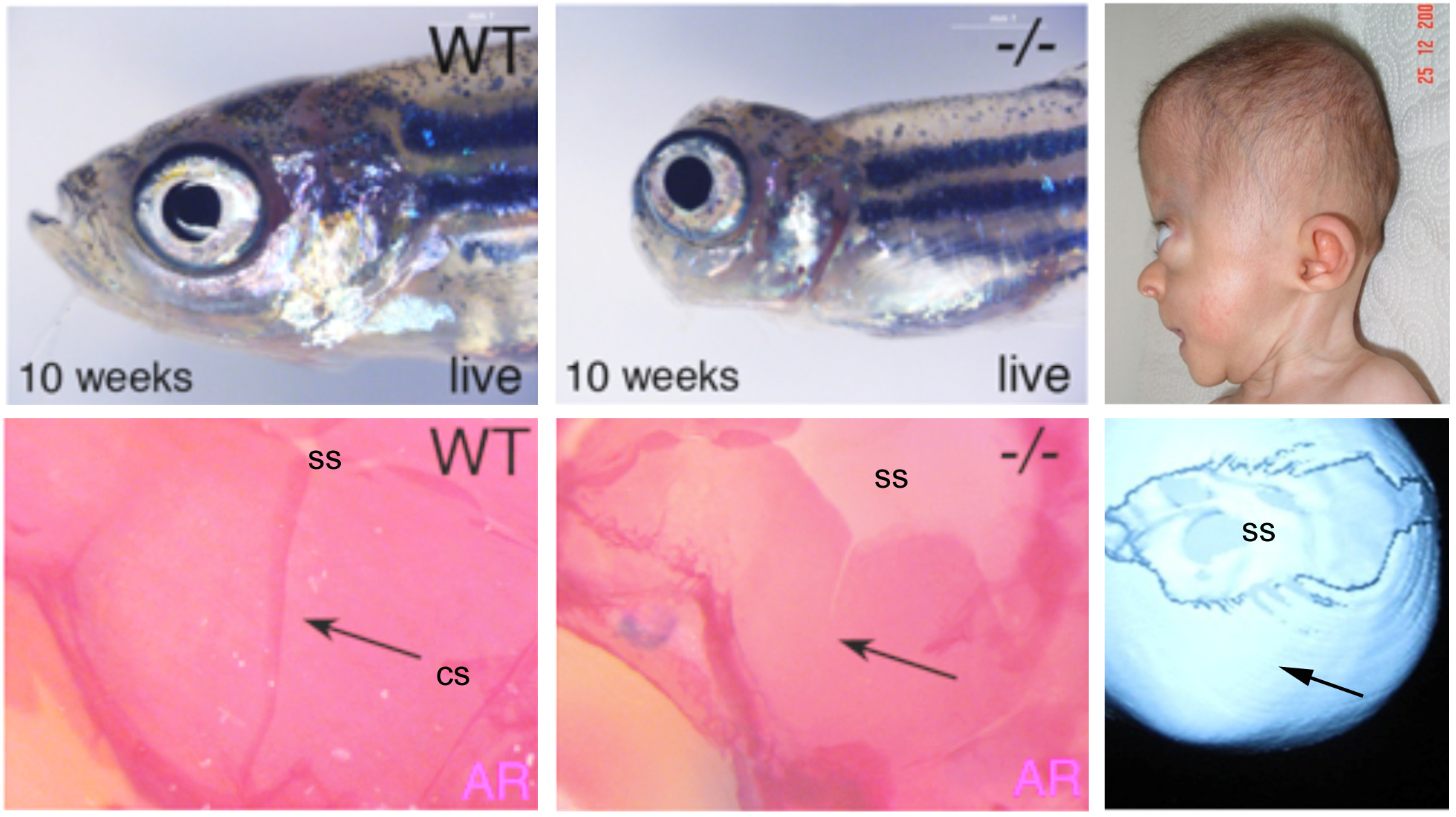
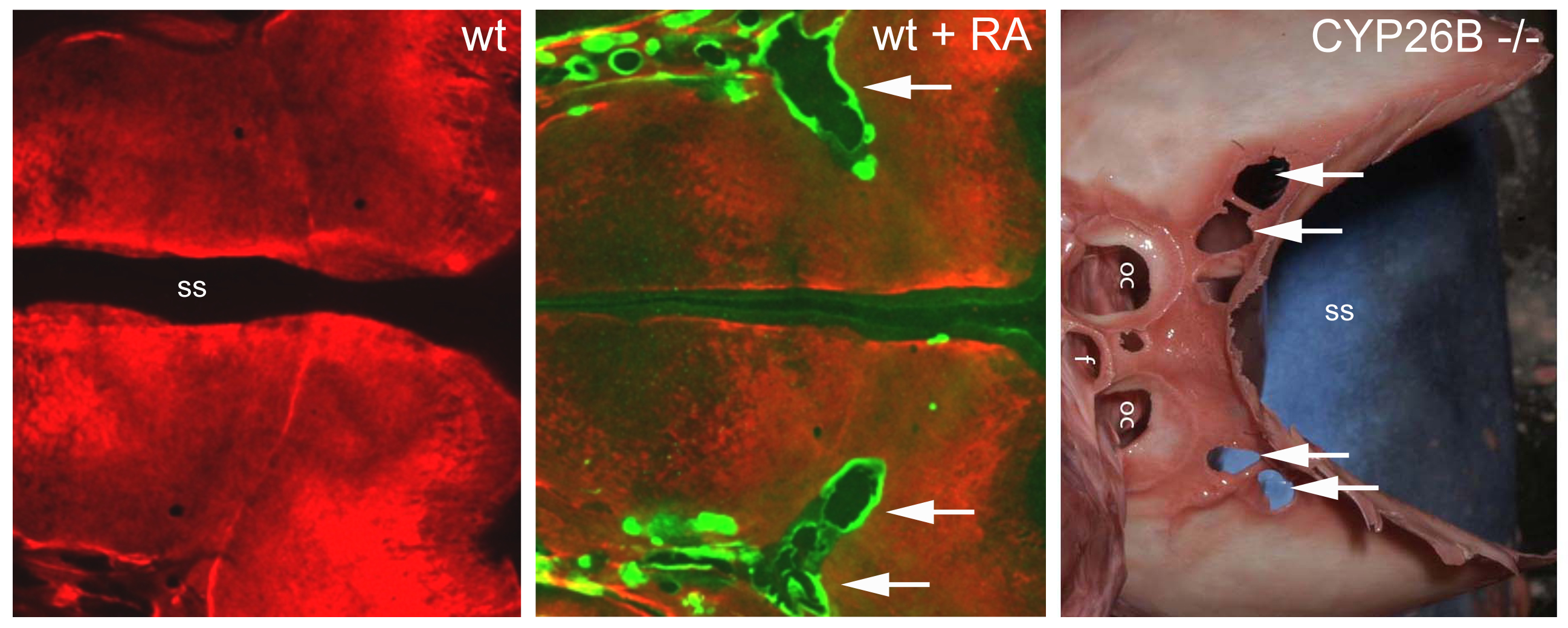
In collaboration with human geneticists, CYP26b1 mutations could also be identified in human patients with severe synostoses (fusion of bones), including fusions of the calvarial plates of the skull (coronal craniosynostosis), as well as calvarial fragmentation at other sites of the skull. Functional studies in zebrafish indicate that both calvarial fusion and calvarial fragmentation, two seemingly opposite effects, are due to an RA-induced precocious differentiation of osteoblasts to pre-osteocytes. Osteoblasts generate the proteins of the bone matrix, while pre-osteocytes stimulate the biomineralization of this matrix. Fusion of calvaria is thus due to an ectopic ossification of the sutures between the clavarial plates, which normally act as joints and growth zones of these flat bones (Laue et al., Am. J. Human Genet. 2011). Calvarial fragmentation, on the other side, involves bone-resorbing osteoclasts, which according to our data are much more strongly activated by pre-osteocytes, rather than osteoblasts (Jeradi and Hammerschmidt, Development 2016). Ongoing studies aim to understand why RA-induced calvarial fragmentation only occurs in regions containing cartilage directly underneath the bony skull.
In addition, we aim to elucidate the mechanisms underlying the metameric organization of vertebra progenitors (centra) along the anterior-posterior axis of the developing vertebral column. Interestingly, the relevant bone-forming osteogenic cells (chordoblasts deriving from the notochord) are not organized in a metameric (segmented) manner, but uniformly present in the axis, while there is direct and consecutive metameric signaling of BMPs (Pogoda et al., Front. Endocrinol. 2023) and RA (Pogoda et al., Development 2020) to chordoblasts. However, the exact cellular sources of the segmentation-initiating signals remain unclear and need to be elucidated. Currently, it is even unclear whether they come from the notochord itself or from adjacent, metamerically organized tissues.
Finally, we are working on zebrafish mutants isolated during our forward genetic screens, which display defects in the adult spine, including scoliosis (curved vertebral column), platyspondyly (flattened vertebral bodies) and intervertebral disc degeneration, severe and rather common, but little understood pathologies in human. Whole genome sequencing is ongoing to identify the causative DNA lesions in the different zebrafish mutants, combined by phenotypic analyses at the molecular and cellular level.