Neuroendocrine control of energy homeostasis, physical activity and somatic growth
In mouse, the primordium of the pituitary gland has been shown to be patterned by antagonistic BMP and Fibroblast Growth Factor (FGF) signaling, offering a second system in addition to the gastrulating embryo (see above) to study the morphogenetic roles of BMPs. This motivated us to investigate pituitary development in the zebrafish. Functionally, the pituitary gland (hypophysis) constitutes a physiological link between the nervous and the endocrine system, receiving inputs from the hypothalamus of the brain, to which it responds by the secretion of different hormones that regulate basic body functions such as growth, reproduction and metabolism. Within the anterior pituitary (adenohypophysis) these hormones are made by distinct pituitary cell types, such as thyrotopes generating thyroid-stimulating hormone (TSH) to regulate the thyroid gland, corticotropes generating proopiomelanocortin (POMC) to regulate the adrenal gland and melanocytes, gonadotropes generating luteinizing hormone (LH) and follicle-stimulating hormone (FSH) to regulate germ cell maturation in the gonads, somatotropes generating growth hormone to regulate somatic growth, and several others. During a forward genetic screen, five loci required for proper growth hormone expression in somatotropes of the adenohypophysis were identified. One of them encodes the Fibroblast Growth Factor FGF3, which is not expressed in the adenohypophysis itself, but in adjacent cells of the ventral hypothalamus, from where it signals to the pituitary to promote adenohypophyseal cell survival (Herzog et al., Mol. Endocrinol. 2004; Development 2004). In addition, it acts as a chemoattractant, guiding regulatory hypothalamic axons as well as blood vessels to the pituitary, thereby playing a central role in the formation of this neurohemal organ (Li et al., Development 2013).
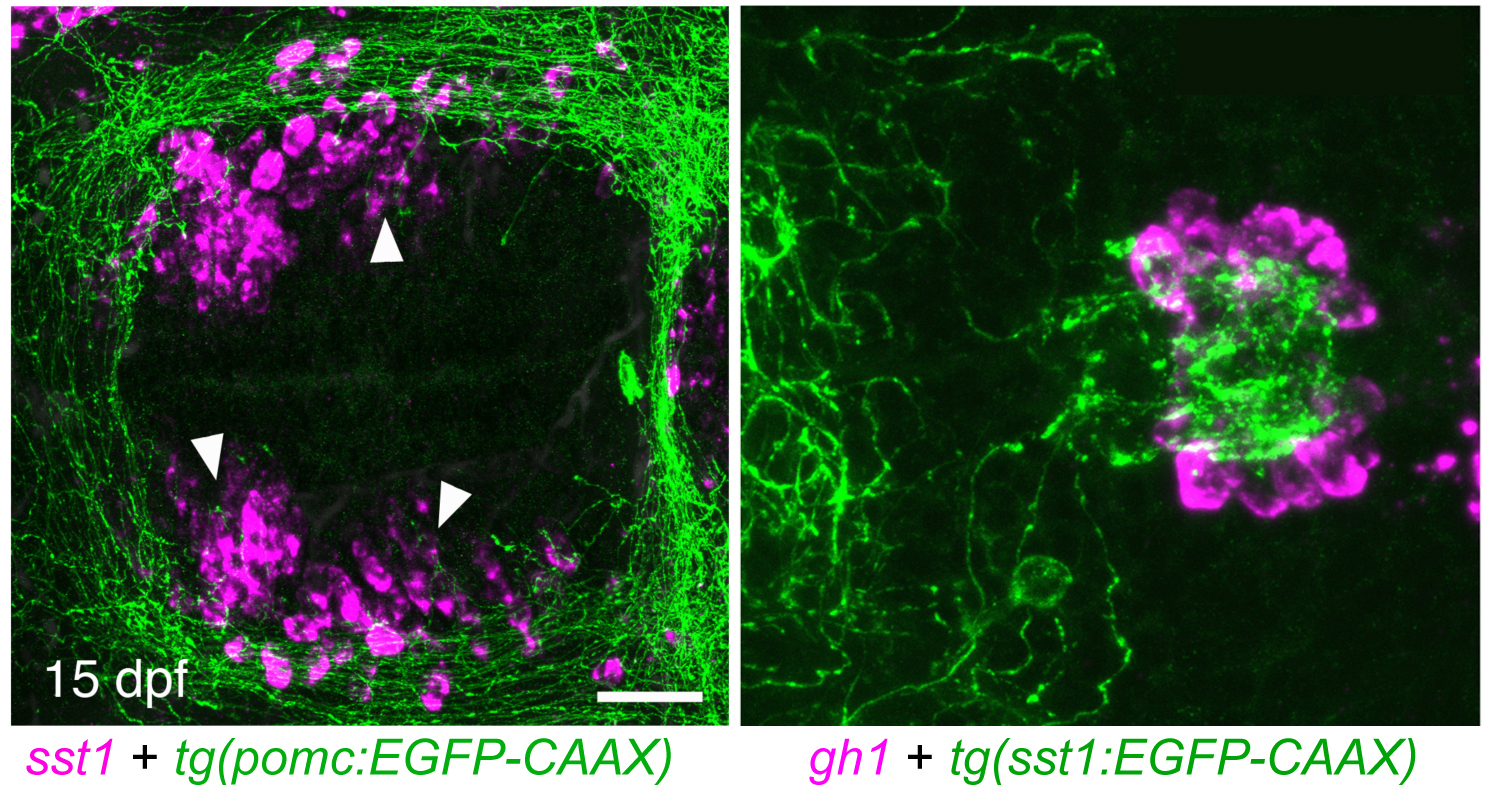
In the meantime, we study pituitary function, rather than its development. In mammals, the activity of the different pituitary cell types is under neuroendocrine control of neurons from the hypothalamus of the brain. Hypothalamic neurons that regulate the activity of corticotropes and thyrotropes of the pituitary gland have been shown to act as second-order neurons of the melanocortin system in neuroendocrine control of energy homeostasis. The primary neuropeptide made by first order neurons of this melanocortin system is alpha-MSH, a cleavage product of POMC, which is generated in the arcuate nucleus of the hypothalamus in response to blood glucose levels and peripheral hormones like insulin, ghrelin and leptin, mediating satiety by binding to its receptor, MC4R, at second-order neurons. Zebrafish also contain a functional hypothalamic melanocortin system (Löhr and Hammerschmidt, Annu. Rev. Physiol. 2011). However, in contrast to the mouse, where loss of MC4R leads to severe obesity, corresponding zebrafish mutants or wild-type fish fed ad libidum or with high-fat diet display only moderate obesity but increased somatic growth. In addition, in females, caloric energy is preferentially spent in ovarian growth and reproduction (Leibold and Hammerschmidt, PLoS One 2015). Even as adults and long after the cessation of somatic growth, zebrafish kept under suboptimal nutrient conditions start to grow again when subjected to ad libidum feeding (manuscript in preparation), pointing to a strong connection between the energy homeostasis system and somatic and ovarian growth. In this light, we hypothesized that hypothalamic Growth Hormone Releasing Hormone (GHRH)- or Somatostatin (SST)-, as well as Gonadotropin Releasing Hormone (GnRH)-expressing neurons, which project to the pituitary to regulate growth hormone and gonadotropin production or release, respectively, are second-order neurons of the melanocortin system. To test this, we have generated transgenic zebrafish in which for instance POMC, SST1 and GnRH are labeled with different fluorochromes to study the proposed hypothalamo-hypophyseal neurocircuits in vivo, allowing us to address the development and plasticity of the system in response to the metabolic state of the animal via live imaging. In addition, functional transgenics have been generated to hyper-activate or ablate the different cell types in a temporally controlled fashion to analyze the impact of these neurons on energy homeostasis, somatic growth and reproduction. Applying these approaches, we could reveal the existence of POMC-SST1-GH melanocortin-pituitary axes which regulates somatic growth of the fish (via growth hormone release) in response to their metabolic state sensed by the POMC neurons. In addition, in collaboration with Prof. Peter Kloppenburg from the Institute of Zoology and Prof. Jens Brüning form the Max-Planck Institute for Metabolic Research in Cologne, we could show that a similar axis, although with less prominent effects, exists in mammals (Löhr et al., Cell Rep. 2018). Together, these studies identified a novel, evolutionary conserved hypothalamo-hypophyseal axis and hypothalamic Somatostatin-expressing neurons as novel POMC targets and thus second-order neurons of the melanocortin system in control of energy expenditure.
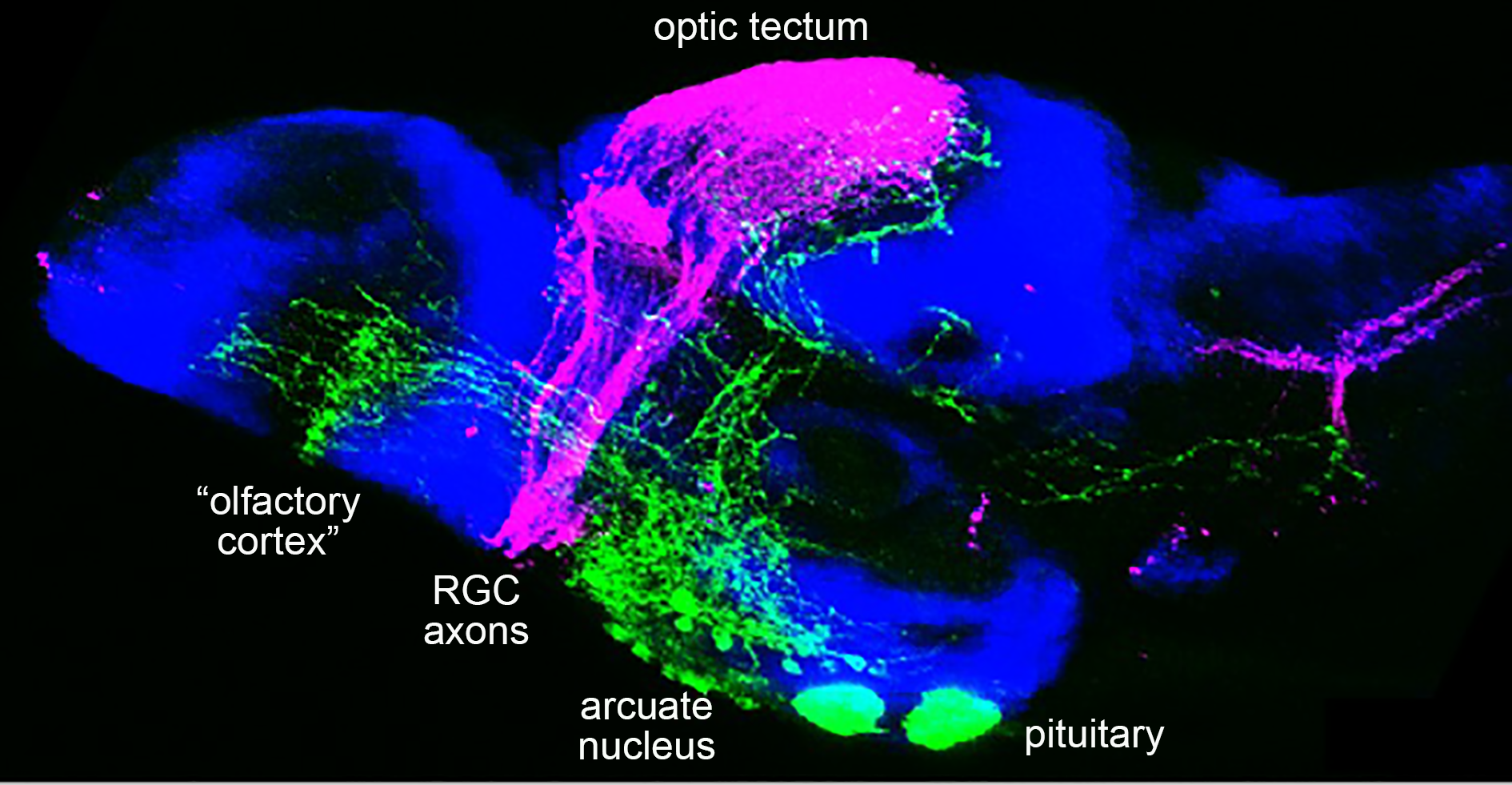
An unexpected finding of our analyses with the transgenic line labeling the axons of the hypothalamic POMC (first order) neurons was their direct projection into the optic tectum, the olfactory bulbs, the hindbrain and spinal cord, suggesting that they might also be involved in regulating different aspects of energy uptake. For spinal POMC projections, we could indeed identify inter- or motoneurons of the spinal cord as novel direct POMC target cells whose excitability is reduced upon ad libitum feeding / satiety and high POMC neuron activity, resulting in attenuated swimming and foraging activity of zebrafish larvae. In collaboration with Prof. Peter Kloppenburg from the Institute of Zoology and Dr. Henning Fenselau from the Max-Planck Institute for Metabolic Research in Cologne, we could further that a similar axis exists in mammals, although in adult mice, satiety and increased POMC neuron activity leads to an increased excitability of spinal neurons and increased locomotor activity. This suggest that this POMC-spinal neural circuitry per se is evolutionary conserved but can have different behavioral consequences in adaptation to different life styles of the animals (Reinoss et al., Curr. Biol. 2020).
Currently, we are studying the roles of the POMC and AGRP innervations of the optic tectum and the olfactory bulb, hypothesizing that they regulate the excitability of neurons processing visual and olfactory stimuli involved in prey sensation. These studies are carried out within the frame of the Cologne Excellence Cluster for Ageing-related Diseases (CECAD) and the Research training group “Cellular and Subcellular Analysis of neurocircuits” (RTG 1960).
Finally, we have carried out forward genetic screens for larger and smaller fish to identify novel regulators of energy homeostasis, obesity and somatic growth. Fish lacking growth hormone have been formerly shown to display decreased body length, but moderate obesity. In our own screens, we have isolated over 20 more mutants with somatic dwarfism of different degrees. The causative mutations are currently identified via whole genome sequencing, and mutants will be analyzed more thoroughly at cellular and physiological levels.